- +86-28-86155036
- sales@scingia.com
- en
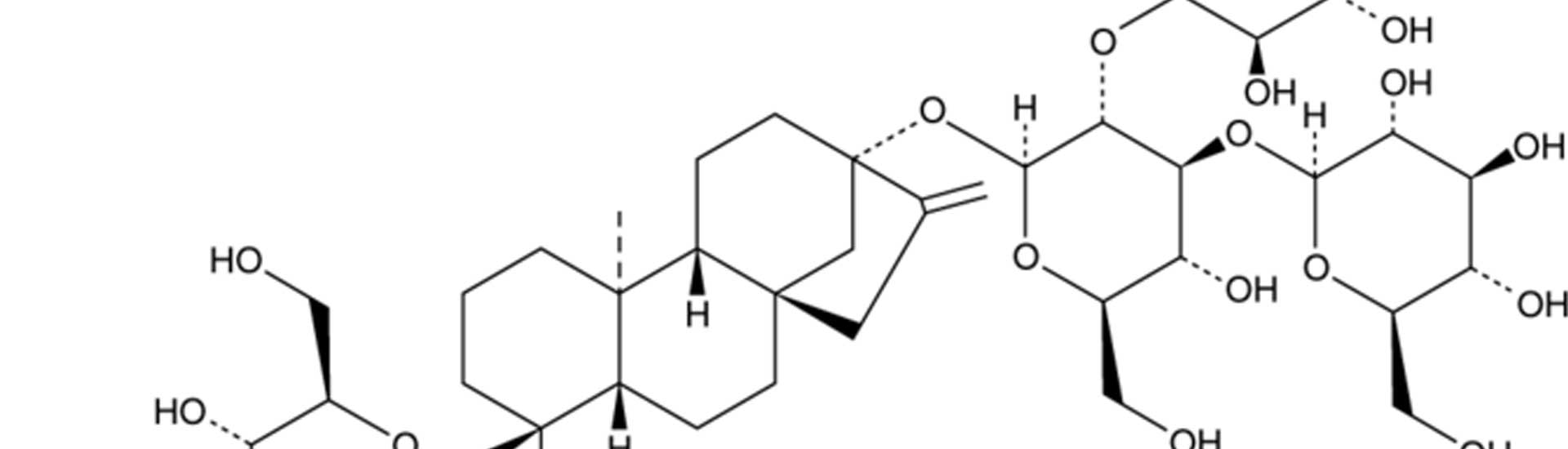
Ingvia® D95 Natural Stevia, which tastes more sugar-like compared to the more common steviol glycoside rebaudioside A, is getting more and more attention from the food and beverage industry. Ingvia® D95 Natural Stevia D has one of the best sweetness profiles of all steviol glycosides. Reb d stevia offers lower bitterness and clean taste, which makes it easier to use.
DETERMINATION | SPECIFICATION |
IDENTIFICATION | |
Color | White fine powder |
State | Powder or crystal |
Identification | Consistent with standard |
Solubility | Freely soluble in a mixture of ethanol and water (50:50) |
ASSAY | |
Total Steviol Glycosides (wt/wt% on dry basis) | ≥95.0% |
Rebaudioside D (wt/wt% on dry basis) | ≥95.0% |
TESTS | |
Loss on Drying | ≤6.0% |
Total Ash | ≤1.0% |
Total Heavy Metals | ≤10mg/kg |
MeOH Residual | ≤200mg/kg |
EtOH Residual | ≤5000mg/kg |
MICROBIOLOGICAL | |
Total Plate Count | ≤1000cfu/g |
Yeast & Mold | ≤100cfu/g |
Salmonella (/25g) | Negative |
| USA (Product Name) | EU(Produce Name) |
| steviol glysocosides | REBAUDIOSIDE D PRODUCED VIA ENZYMATIC CONVERSION OF HIGHLY PURIFIED REBAUDIOSIDE A STEVIA LEAF EXTRACTS (E 960c(iii) ) |
For detailed Reb D stevia labeling usage rules, please contact our staff by email: zhanglei@scingia.com
Certification |
| HALAL, Kosher, ISO 22000:2018 FSSC 22000 (5.1), FSVP, Non-GMO, Organic |
◉ Statement of Ingvia® D 95
◉ Aflatoxin Free
◉ Animal Free
◉ Benzo[a]pyrene and P.A.H Free
◉ BSE-TSE Free
◉ ETO Free
◉ GE3-MCPD Free
◉ Gluten-Free
◉ Glyphosate Free
◉ Latex Free
◉ Nanomaterials Free
◉ PHOs Free
◉ Phthalates Free
◉ Pyrogens Free
◉ Sewer Sludge Free
◉ Steroids Free
◉ Sulfite Free
Non-GMO Available
Kosher
Halal
Hypoallergenic
Gluten-Free
Organic Available