- +86-28-86155036
- sales@scingia.com
- en
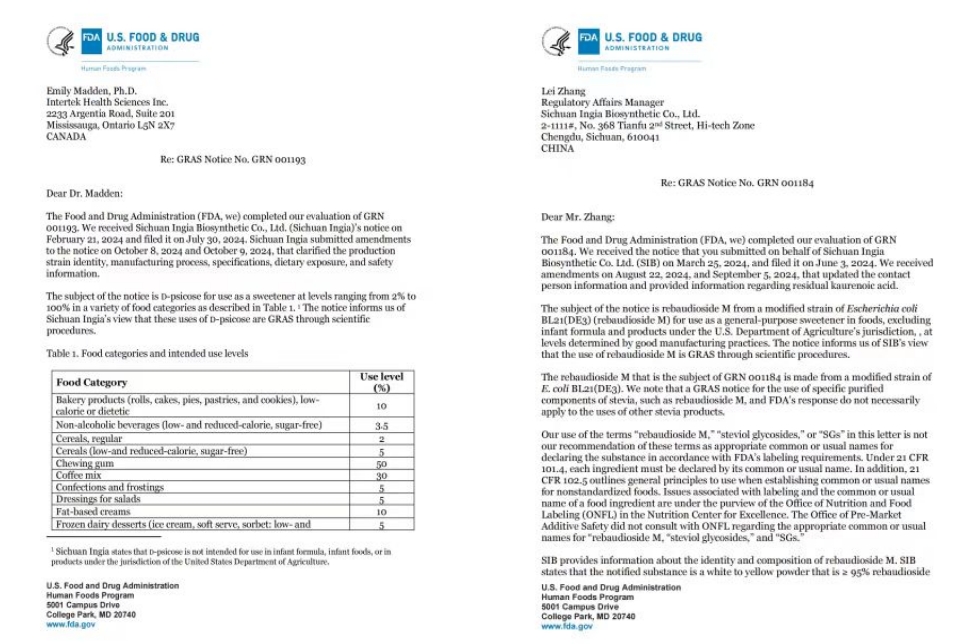
We're excited to share that INGIA has secured FDA GRAS (Generally Recognized As Safe) status for its innovative Fermented Steviol Glycosides Rebaudioside M and Allulose, marking a significant step forward in the natural sweetener industry. This accomplishment makes INGIA the first Chinese company to earn GRAS for Fermented Rebaudioside M and the third for Allulose in China. With this, INGIA now holds a total of 5 FDA GRAS certifications.
**Rebaudioside M** is celebrated for its exceptional sweetness and near-zero calorie content, making it a favorite among health-conscious consumers. **Allulose**, on the other hand, is a low-calorie rare sugar that tastes almost identical to sucrose, participates in the Maillard reaction, and has the added benefit of not spiking blood sugar levels. As the demand for "zero-sugar" and "reduced-sugar" products continues to grow globally, the shift from traditional "petrochemical-based" to "bio-based" products is accelerating. This trend opens up enormous opportunities for natural sweeteners produced through bio-manufacturing, with biology offering endless possibilities to expand the range of natural "sweet elements."
Bio-manufacturing natural sweeteners not only frees production from the constraints of soil and climate but also ensure that consumers worldwide can enjoy delicious, affordable, and natural sweetening options. At INGIA, we're proud to lead this transformative journey, using advanced biotechnology to create a sweeter, healthier future for everyone.
This is the first one.